First patient for artificial heart dies
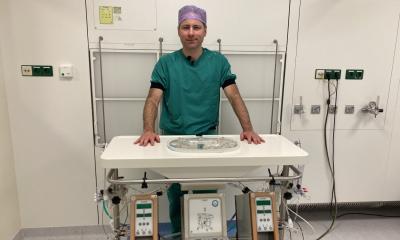
The first patient to receive a totally implantable artificial heart died 75 days after the procedure. The cause of death on March 2, 2014 was not disclosed in a short announcement made by the Hôpital Georges-Pompidou in Paris. European Hospital reported on his new heart device in the recently published issue 1/2014.

The 76-year-old patient, who has not been identified, was "fully aware of the risks and by his confidence, his courage and his willingness has made a remarkable contribution to the efforts undertaken to combat a rapidly progressing disease," the medical team stated.
The doctors said they wish "to highlight the value of the lessons learned from this first clinical trial, with regard to the selection of the patient, the close monitoring, the prevention and treatment of difficulties encountered."
The most recent report on the patient's health in late February said the patient was able to feed himself and no longer required respiratory assistance. He was described as a big man with a great sense of humor who less than a month after the operation was passing his time watching television, listening to the radio and receiving visitors.
At that time the lead physicians predicted the recovery would be long and difficult, but in many aspects little different from that of any patient of the same age and the same pre-operative condition.
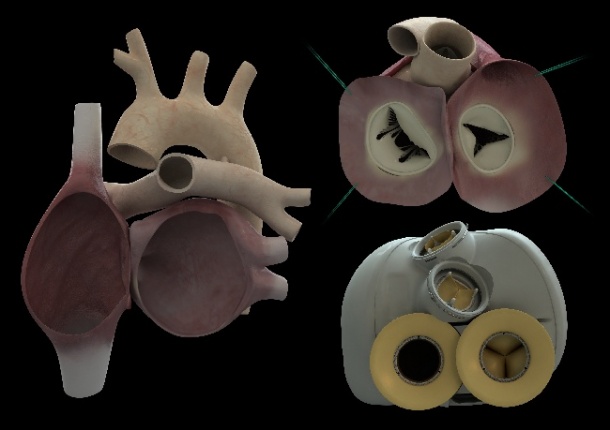
The 900-gram heart was implanted Dec. 18, 2013, by a surgical team led by Christian Latremouille, M.D., with the participation and guidance of the inventor of the device, Alain Carpentier, M.D. It was the first time an artificial heart requiring no external pumps had been implanted. Only two wires exited the body at the abdomen, one to supply power and a second to monitor device performance. It is also the first artificial heart capable of adapting the blood supply according to a patient's activity, varying from three to nine liters per minute, rather than a constant supply.
Carmat, the manufacturer of the artificial heart based in Paris, said in a statement it "wishes to pay tribute to the courage and the pioneering role of this patient and his family, as well as the medical team's dedication."
Three further implantations of the Carmat heart are authorized by French health authorities, and it was not known if the death of the first patient would put into question those operations.
"Wait for the results of the autopsy," advised Jean-Noël Fabiana, head of cardiovascular surgery at the Hôpital Georges-Pompidou, according to the French daily newspaper, Le Parisien.
A rejection of the heart device by the patient's body is considered out of the question by Carpentier, and is expected to be one of the advantages of the Carmat prosthesis as this will reduce the number of medications typically required for patients receiving a human heart transplant. The device was developed over 25 years using only parts made of materials already validated as bio-compatible in collaboration with engineers at the European Aeronautic Defence and Space company (EADS).
Carmat was created by EADS, the Foundation Alain Carpentier and Truffle Capital of Paris and became a public company in 2010.
In an interview, Carmat CEO Marcello Conviti told European Hospital the key clinical indicators being monitored during this first-in-man trial phase of clinical evidence were "of course, survival. Then there are all the of physiological indicators of blood delivery to the brain and liver, whether the pumps are providing the body with enough blood to allow all the organs to recover and work properly."
Carmat estimates around 100,000 patients in the United States and Europe could benefit from its artificial heart. Conviti estimated the market value for end-stage patients eligible for the implantation to be worth more than $1.5 billion.
In this first phase of clinical development Carmat will follow four French patients for six months, and "with good results we will be able to apply for the CE mark, which we hope to have by early 2015," Conviti said.
In this test for safety and efficacy, "we are dealing with high-risk, end-stage patients", he emphasized, adding that a second trial phase for device performance with up to 20 patients with less severe conditions than the first four French patients would follow in Germany, Belgium, Italy, Poland, and Saudi Arabia.
For a complete story on this new heart device, see "Replacing Human Hearts" in the first edition of European Hospital in 2014.
04.03.2014