Network science
Discovering what causes diseases
Systems biology allows the mathematical visualisation through graphs and networks of complex body processes such as disease development. The aim is to improve understanding processes and triggers of diseases, so as to access and repair a damaged network. ‘We are still approaching this issue with a lot of naivety and underestimate the complexity of biological systems, and therefore of diseases,’ says Professor Rudi Balling, Director of the Luxembourg Centre for Systems Biomedicine at Luxembourg University.
Report: Marcel Rasch

‘Network Science in a biomedical context is derived from the concept of a system,’ Professor Rudi Balling explains. ‘If you look at any system you will find certain similarities that will occur over and over again. There are components that react with one another and we can look at this interaction over time and observe the dynamics. All of this can be described mathematically with graph theory as a network.
‘Transferred into the world of biological systems, organs and cells, and lastly also diseases, this means that we can also record the different cell types and different relations between the cell types as graphs or networks respectively. ‘If we, for instance, look at a liver tumour or inflammation, we can see where the relationships between the different components change. These changes can then also be visualised as graphs and be compared with one another mathematically. We try to describe and understand diseases as changes in networks.’
Challenges
‘In the world of medicine and biosciences this is our first attempt at systematically and (almost) completely capturing all components. The main challenge currently is of a purely technical nature. Currently, capturing the genome sequence is no longer a problem; but capturing all proteins quantitatively is still a big challenge. Each organ produces its own, different set of proteins, and therefore the genome is read organ-specifically. Measuring this is very difficult.
‘There is also a kind of “background noise” in the body that fluctuates from hour to hour, minute to minute and from person to person. Unfortunately at the moment, we don’t understand what level of fluctuation is “normal” and what level may be an indication of disease, which is gene-controlled in respect of the adaptation of a certain organ. Even more important is the understanding of control cycles, such as blood pressure and blood glucose. Why does the body change the control variable of blood glucose, which makes obese people more prone to developing diabetes? We need to do more research here.’
‘The human body has around 20,000 genes that can probably code 100,000 to 300,000 different proteins. The possible combinations are therefore almost infinite and we have not yet discovered which combinations nature actually utilises and under which conditions. Our ultimate objective is to intervene in a damaged network to repair it.’
An ideal approach
‘It would be ideal to carry out family studies. On average, we would have to examine 30,000 to 40,000 patients to obtain relevant data that could point to a disease or its development. However, if we examine families with known hereditary diseases, then it suffices to sequence just one family to find the path of inheritance and the responsible gene. We need to get to the depths of things.
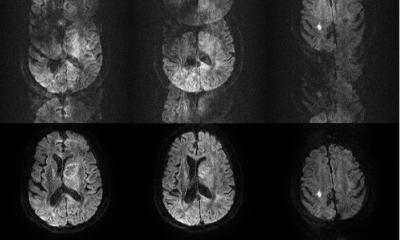
‘We are also currently developing Spatial Systems Biology where, in addition to temporal systems biology, we are looking at the spatial resolution. Thanks to modern imaging procedures and new, high-resolution microscopes we can see how a cell works, when, where and which proteins move in, which way and what they do exactly. We try to model and predict these dynamics.’
The objective
‘We want to observe what happens when mutations occur, or what changes when we change different parameters. In the future, we will work mainly with non-invasive imaging procedures that have an ever-improving resolution. This allows the combination of different procedures, which is very interesting. However, in doing this we always produce an enormous volume of data – in future likely to be in the region of terabytes of data per second. Therefore, we either need better storage solutions or we’ll have to analyse in a more sophisticated way.’
More to come
‘We’ll have to have in-depth discussions around the issue of data protection. In the era of social media, is the data protection law not actually well beyond what is required? As a society, we don’t yet know how to deal with this, but we’ll have to find a way. The discussion around data protection is anyway a lot more intensive in Germany than in other countries. Unfortunately, this data protection law is a hindrance in certain ways, not only for research but for patients as well.
‘The discussion around utilisation of the Cloud should also be carried out in a different way. In the future we will have few alternatives to the use of the Cloud, because we won’t be able to efficiently store and transport data in another way. ‘Institutes can no longer cover the storage costs of our own data on internal servers. It might work up to the terabyte and petabyte level, but from the exabyte-level onwards it will no longer be possible.
‘The real question should therefore be: Do we stop research or will we get secure Clouds? This development will have a dramatic impact on the next generation because biomedicine cannot work without mathematics – it will therefore have to work safely with IT and large data volumes.’
PROFILE:
Rudi Balling studied nutrition at the University of Bonn and Washington State University (USA) and gained a PhD in Human Nutrition from the University of Bonn in 1984. After several research posts, in 1993 he was appointed Director of the Institute of Mammalian Genetics at the GSF National Research Centre for Environment and Health in Munich. Then, in 2001, he joined the Helmholtz Centre for Infection Research in Braunschweig as its Scientific Director. Eight years later, Prof. Balling became the founding director of the Luxembourg Centre for Systems Biomedicine (LCSB).
10.09.2015