Pain relief
Medasense Launches New Pain Monitoring Device, PMD200TM following European CE approval
New state-of-the-art technology helps clinicians provide personalised pain relief
Medasense Biometrics Ltd. announced today that it has received CE mark approval for its novel Pain Monitoring Device, PMD200. This new technology is now available to help physicians objectively assess a patient’s pain in critical care situations, where patients are unable to communicate. This allows physicians to ensure pain is properly managed.
Objectively assessing how much pain an individual person is in has long been a challenge. When a patient is unable to describe their pain, it is even more of a problem. There are currently no validated objective markers of pain that are recommended for clinical use1 and this can make it difficult, especially for anaesthesia teams, to provide the right amount of pain-relief medication.
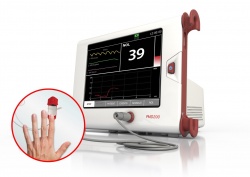
To address these challenges Medasense has developed PMD200, a pain monitoring device based on the patented NOL™ technology that quantifies patients’ physiological response to pain. The easy-to-use system consists of a non-invasive finger probe which acquires physiological signals from four different sensors and calculates dozens of pain-related physiological parameters. This data is then analysed by artificial intelligence algorithms and converted into a single pain index, the Nociception Level (NOL) index, where 0 = no pain and 100 = extreme pain.
Using the system will enable physicians, in particular critical care teams, to optimise and personalise pain treatment avoiding over or under use of pain medication that can result in significant complications2,3. On regaining consciousness after surgery, common complications resulting from opioid administration include nausea, vomiting, respiratory depression, constipation4 and hyperalgesia5. Successful clinical validation studies in world-class European and Canadian hospitals support its use in these settings, with the NOL™ Index demonstrating superior intraoperative pain assessment over currently used methods6,7.
Professor Albert Dahan, MD. PhD. from the Department of Anaesthesiology at Leiden University Medical Center in the Netherlands said, "We have been studying the PMD device for a number of years now, and I believe that the NOL index may allow for more balanced anesthesia, as for the first time we are able to titrate analgesic medication to patients' needs. In the upcoming weeks the LUMC will be adding PMD200 devices into the operating rooms, in the future I hope to see the NOL index integrated into other monitors as it provides significant decision support information and can potentially positively impact patient outcomes".
“The European launch of PMD200 marks a significant milestone for Medasense’s breakthrough pain assessment technology,” said Galit Zuckerman, Medasense’s CEO and founder, “With future products and applications in development, our technology has the potential to dramatically change pain care, pain research and the industry as a whole.”
The company is setting up distribution networks to make the system available in operating rooms and critical care units across Europe.
The company is also conducting research in other forms of pain, for example chronic pain including long-term back pain, with the aim of broadening the situations where the NOL Index could help with pain management.
References:
- Cowen R et al., (2015) Assessing pain objectively: the use of physiological markers. Anaesthesia, 70:828-847
- Gan TJ et al., (2014) Incidence, patient satisfaction, and perceptions pf post-surgical pain: Results from a US national survey. Current Medical Research and Opinion. 30:149–60
- Pogatzki-Zahn E et al., (2015) A Prospective Multicenter Study to Improve Postoperative Pain: Identification of Potentialities and Problems. Plos One, 10:e0143508
- Benyamin R et al., (2008) Opioid Complications and Side Effects. Pain Physician: Opioid Special Issue. 11:S105-S120
- Joly V et al., (2005) Remifentanil-induced postoperative hyperalgesia and its prevention with small dose ketamine. Anesthesiology, 103:147-55
- Julien M et al., (2016) Nociception Level (NoL) index alteration after standardized nociceptive stimulus decreases with higher doses of remifentanil. Abstract:European Society of Anaesthesiology.
- Edry R et al., (2016) Intraoperative validation of the NOL Index, a non-invasive nociception monitor. Anesthesiology, 125:193-203
Source: medasense press release
Media Enquiries
Kate Perry
Phone: +44 (0) 7747 533 260
Email: kperry@jpa.com
30.01.2017