ESC roundup: Fixing heart conditions
Devices to treat chronic cardiac disease are winning credibility with new evidence from large-scale patient registries, John Brosky reports
With a record 32,946 participants, the annual congress of the European Society of Cardiology this year became the largest cardiology meeting in the world.
Traditionally oriented to pharmaceutical therapies, this year medical devices gained ground against drugs with successful outcomes for treating chronic conditions, such as arrhythmias, blocked arteries or deteriorating valves.
At past ESC meetings such studies were typically from manufacturers with highly selective patient populations and narrowly focused on device performance. ESC 2011 marked a turning point with the emergence of doctor-driven studies based on real world patient registries with a single goal, to know whether patients get better or die.’ This is the year for registries,’ proclaimed the new ESC president, Michel Komajda MD, proud of the success of the society's effort to introduce wider clinical evidence for devices.
Better outcomes with new generation stents
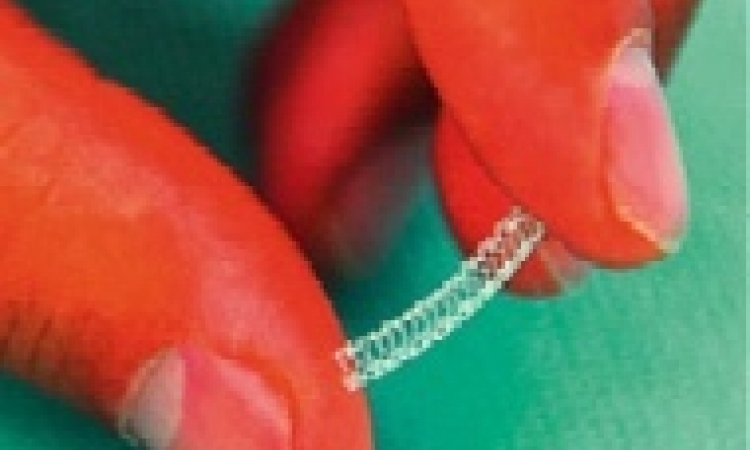
Placing a metal sleeve inside an artery to keep it open became a widespread practice in Europe until experience showed the body, reacting against this unnatural device, would close up the artery again.
Manufacturers responded by coating the tiny tubes with a drug to prevent this restenosis. Sales of drug-eluting stents (DES) then soared until 2006, when the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) showed they simply did not work.
SCAAR was back at ESC this year, this time to show that, with third generation DES, manufacturers have finally got it right.
Results of 94,384 stent implantations in Sweden, from November 2006 to October 2010, showed the new DES are associated with a 38% lower risk of clinically meaningful restenosis and a 50% lower risk of stent thrombosis compared to old generation DES. Significantly, after 15 months, patients with the new DES no longer have adverse effects.
A Japanese study based on ESC-developed methods reminded cardiologists that, for complex cases where several arteries are clogged, open heart surgery is still the better treatment. The CREDO-Kyoto registry of 2,981 patients with triple vessel disease showed bypass surgery had a ‘protective effect’ against future cardiac events while the risk for all-cause death was significantly higher for stents.
A study from western Denmark showed a coordinated strategy to rush patients directly to a cath lab for stenting saves lives and improves long-term outcomes.
A registry analysis for 9,514 patients demonstrates field triage by emergency medical teams saves one hour over hospital processing, resulting in lower mortality and lowering the risk of repeat adverse events.
ICD home monitoring does not reduce mortality
Patients at risk of sudden cardiac death as a result of rhythm disturbances routinely receive an implantable cardiac defibrillator (ICD).
In Western Europe the average rate of implantation is 140 ICDs per one million people, far below the shocking utilisation in the USA, where 600 ICDs are implanted per million people.
Because a cardiologists' office is quickly overwhelmed by the required follow-up visits every three months, remote monitoring features were introduced to ICDs.
Studies show home monitoring devices are safe and help reduce the office workload. But does automatic reporting from the device reduce adverse events and lower costs for a healthcare system? The short answer is no.
Two French studies presented at ESC are among the first to challenge the cost-benefit of ICD home monitoring.
France has one of the lowest implantation rates among developed countries with just 88 ICDs per million people. Before encouraging wider use of these devices, the French Ministry of Health asked for proof that remote monitoring is worth the added expense.
The EVATEL study, paid for by the ministry, revealed that remote monitoring does not affect the rate of major cardiovascular events, showing no difference in survival and hospitalisation at one year between a monitored group and a control group.
The ECOST trial, sponsored by Biotronik followed 433 French patients for 27 months and reported a clear benefit for home monitoring with a 72% reduction in the risk of hospitalisations related to inappropriate shocks.
Unfortunately, the financial impact from the two studies was not reported, because the French ministry is still studying the results.
The difference in findings was explained by the fact that ECOST looked at one device with a smaller patient group, whereas EVATEL is the first trial to include all manufacturers across a large population of 1,500 patients.
Heart valves: Still viable after four years
ESC was the battle ground for studies on transcatheter aortic valve implantations for many years. Because these devices are now in front of the USA’s Food & Drug Administration, the focus has moved to the other side of the Atlantic.
Yet, European cardiologists hold an important edge with long term results among 30,000 patients who have received these devices.
The open question is: How long do the valves last?
The strongest real-world analysis is the FRANCE II registry, with 2,419 consecutive patients from 33 centres in France, which includes the only two devices approved for implantation -- CoreValve from Medtronic and the Sapien valve from Edwards Lifesciences.
Procedural success was reported as excellent and the valves are performing well.
The German Heart Centre in Munich reported on 393 consecutive patients receiving a CoreValve between 2007 and 2011. The device shows sustained improvement of haemodynamic values up to three years after implantation.
A small study of 50 CoreValve patients from seven centres in Europe and Canada is unique for having the longest follow-up period. Receiving a valve in 2005 and 2006, more than half of patients have since died, but not as a result of device failure and the valve continues to perform well for the survivors.
A review of 177 patients implanted with the Edwards valve in Rouen, France, starting in 2006, also showed solid device performance.
Electronic 'nose' smells heart failure
Vasileios Kechagias PhD, from the University Hospital Jena, believes a new device can sniff out the potential for heart failure.
Emphasising that the results are from a pilot study and the device is far from finished, Dr Kechagias said the study succeeded in demonstrating among 126 patients that the ‘electronic nose’ detected heart failure with 89% sensitivity and 88% specificity.
The device consists of metal oxide based gas sensors, each with a different sensitivity to various odorant molecular types.
The first version of the nose is a bulky cube that is strapped to the forearm, and the detection session takes 30 minutes. It also needs to be connected to a high-end gasometer and molecular analysis system at the University for Applied Sciences in Jena. Nonetheless, the potential is intriguing with the possibility of one day seeing a miniaturised version for a skin patch that remotely transmits the readings.
‘Sometimes it takes something crazy to shake up our ideas,’ said ESC session chairman Frank Ruschitzka MD, from the Zurich University Hospital.
20.10.2011