Multimodal visible embolisation particles
Embolisation – the blocking of vessels - is a key procedure in Interventional Radiology. It plays a steadily growing role in the treatment of various tumour lesions, with hepatic cellular carcinoma and uterine fibroids the main focus.
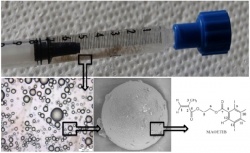
syringe ready for injection. Microscopic
and electron microscopic images of the
multimodal particles are shown along the
chemical structure of the core polymer
Different embolisation materials have been developed over time, which vary in size and chemical composition. For TACE (trans-arterial chemo-embolisation) embolisation materials are combined with chemotherapeutics, which are slowly released into the target lesions.
All currently clinically available embolisation particles share one feature: they are not visible in any imaging modality. Thus direct visibility of the material would provide several advantages, especially combined with 3-D imaging techniques that are increasingly available in modern catheter labs.
With direct visible embolisation, material misplacement of embolisation agents could be far better detected than today. Shunting or ‘spillage’ of particles may be recognised and prevented on the fly. Direct visibility would also allow the clear positioning of embolisation materials within the tumour tissue - tumour re-growth could be correlated to embolised areas. All this might open up new opportunities to refine embolisation therapy and to develop personalised therapy concepts, which are not possible today.
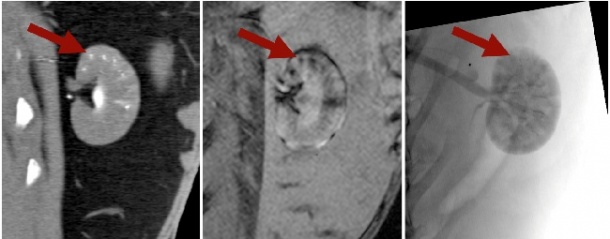
In the past, within basic research, embolisation particles were described that were visible within one imaging modality (X-ray or MRI). We out-performed these developments and synthesised the first embolisation particles, which are visible within more than one imaging modality. They can be visualised in X-ray based fluoroscopy and computed tomography (CT) and detected in radiation free magnetic resonance imaging (MRI).
The interventionalist would therefore be in a comfortable position to change imaging modalities intra-procedurally, without losing the ability to visualise the particles. This could be of immense interest in the case of uterine fibroid embolisation, where radiation dosage is critical. Here, in a multimodal setup, catheter placement could be performed within the angiographic setup, while the most radiation to sensitive organs is caused by the embolisation process, which could then be performed in the MRI site.
Using different imaging modalities is also of interest regarding follow up examinations, which can be done in MRI while the embolisation process itself can take place in conventional X-ray based angiography. This would significantly increase the acceptance of long-term follow-up studies. Follow-up examinations could provide new insight and help to understand and improve embolisation therapy with the long term of new personalised approaches to embolisation therapy.
Embolisation particles consist of a polymerised iodine core with USPIO (Ultra-small paramagnetic iron oxide particles) on the outside. Both substances are approved and in daily use in clinical practice with a good long-term safety profile. Thus, the design of our particles will hopefully increase acceptance among physicians and patients and might decrease approval hurdles.
They can be created in various sizes (40-200 μm) and could easily be combined with different therapeutics (Image 1). However, research to define exact loading capabilities is currently in progress. Early animal experiments were successful (Image 2).
A standardised and flexible production process will be implemented by Fraunhofer IPA in the Reis group. The particles will be produced in standard quality and defined bandwidths of particle size.
The particle production will be set up in a way that GMP compliance is followed. IP was secured and the transition to clinical practice has begun with commercial partners.
Authors: S H Bartling, J Budjan, M Sadick, H Aviv, S Margel, C Reis, S Diehl, from the Institute of Diagnostic Radiology and Nuclear Medicine, University Medical Centre, Mannheim, Germany; Department of Chemistry, Bar-Ilan university, Ramat-Gan, Israel and the Production and Process Automation Department, Fraunhofer Institute Manufacturing Engineering and Automation
26.10.2011