Sponsored • Screening for the use of 5-FU anti-cancer drug
Fully-automated DPD deficiency testing
Each year almost 80,000 new patients in France alone receive fluoropyrimidines, a group of anti-cancer drugs including 5-FU which is normally administered intravenously to treat digestive, breast and head and neck cancer.
Interview: Daniela Zimmermann
However, fluoropyrimidines-based chemotherapies can cause severe toxicities (incidence at around 20%) and sometimes lethal toxicity (incidence between 0.1 and 1%) with part of these toxicities possibly related to deficiency in the activity of the main enzyme enabling elimination of 5-FU, dihydropyrimidine dehydrogenase deficiency (DPD). Because of this risk, routine screening for DPD deficiency before treatment with fluoropyrimidine is now necessary. Healthcare in Europe asked Stéphane Moreau, who is responsible for LC/MS in Europe for the Japanese instruments and medical equipment manufacturer Shimadzu Corporation, to outline what is available for this kind of screening and any new developments.

Two methods currently exists to diagnose DPD deficiency, he said, but underlined that each involves a significant amount of manual work. One is genotyping, though key to that is in knowing the different variants to detect. ‘It‘s not 100 percent accurate,’ Moreau observed, ‘because you detect only what you know.’
The second technology is LC-MS/MS, which has the advantage that the ratio of uracil and Dihydrouracil is measured directly. ‘As uracil is metabolised to UH2 by DPD, the ratio UH2/U reflects the DPD activity, thus indicating a risk for the use of 5-FU drugs. In this way, you can identify the DPD deficiency, even if you don’t know the different genes influencing the DPD deficiency.
‘In genotyping,’ Moreau added, ‘you are looking for the genes that are the cause of the deficiency while, with LC-MS/MS, you estimate the activity of DPD by measuring the ratio of compounds UH2 and uracil. In one case you look at the cause, in the other, you look at the result. The advantage of measuring the deficiency in DPD via the ratio U/UH2 is that you will take care of all deficiency cases, thus eliminating the risk of toxicity. In that way, this is safer.’
However, he did acknowledge that the problem with LC-MS/MS is that sample preparation is ‘quite tedious, with a lot of manual steps’ and has been limiting penetration of LC-MS/MS.
Recommended article

Sponsored • Chemotherapy
5-FluoroUracil: Curing or killing the patient?
Fluoropyrimidine-based chemotherapies are widely used, at least in the European area, for treating various types of cancer including digestive, breast, throat and brain cancer. Fluoropyrimidines are a group of anticancer drugs including the well-known 5-FluoroUracil (5-FU) and the prodrug capecitabine.
The fully-automated LC-MS/MS method
Potentially leading to toxicity and bone loss by reduction of 5-FU metabolism, DPD has a big impact on the use of the cancer drug 5-FU (5-fluorouracil). Thus DPD identification is key to identifying those patients who may be susceptible to a negative reaction to 5-FU and help to avoid that serious reaction.
Additionally, with the ever-increasing number of tests needed, automating this process has grown in importance. Fortunately, Shimadzu Corporation has now developed a process that promises to speed up testing and also deliver greater accuracy, safety and standardisation. The answer has been to develop a fully-automated LC-MS/MS method for uracil and dihydrouracil in human plasma, known as indirect phenotyping.

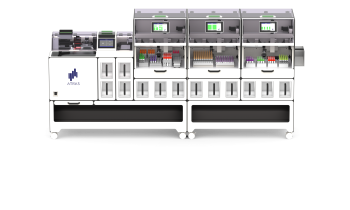
Previous LC-MS/MS methods encountered problems with complex liquid/liquid or solid-phase extraction procedures. However, Moreau explained that Shimadzu has developed a method in which the extraction is carried out by a programable liquid handler directly coupled to a liquid chromatograph (LC) MS/MS system. Specifically, the extraction procedure is performed by a CLAM-2030 coupled with a LCMS-8060 triple quadrupole mass spectrometer.
Now, with the increasing number of tests required – and the new French guidelines introduced in December 2018 that state that all patients treated by 5-FU should be checked for DPD enzyme deficiency – coping with demand and throughput is a challenge, due mainly to the long sample prep process.
Due to the complexity of liquid extraction and the manual steps, Shimadzu received a request from a customer using its CLAM robot to develop a method to automate the preparation process prior to LC-MS/MS. ‘That completely automated this,’ Moreau said, ‘and reduced the analysis time with a solution for the customer to increase the throughput in their lab.’
France has now installed its first systems
Recently validated at a hospital in Limoges, the first systems in France are being installed. Moreau believes this will attract widespread interest in other countries. He points to a presentation, last June at the European Association of Pharmacology and Toxicology in Sweden, from scientists in Leiden regarding their systematic genotyping process with a profile for each patient, but also showing that, with this, there are still cases of toxicity because they do not know all the variants that cause this problem.
‘So, I’m sure LC-MS/MS is the best solution and that automated sample prep will help a lot of people.’ The automated process is time-saving, improves workflow and offers stan-dardisation. The Shimadzu LC-MS/MS approach has cut manual sample prep time from 30 to 10 minutes or less, but a major benefit is that the next sample is being prepared while the existing sample is being run on the system, increasing throughput. Sample analysis is also reduced to 15 minutes. After the sample is placed on the robot there is no need for human manipulation and, as the robot handles every sample in exactly the same way, standardisation is high.
With so many cancer patients receiving 5-FU-based chemotherapy, Moreau stressed that the importance of finding out quickly about DPD deficiency is now widely recognised and he believes the Shimadzu solution of automating sample prep and a LC-MS/MS method to measure uracil and dihydrouracil with no human intervention once the sample tube is positioned, is set to play a key role in speeding up this process, and offer better and quicker outcomes for patients.
Profile:
In 1994 Stéphane Moreau obtained his diploma from INSA in fine chemistry and engineering with a specialisation in chemical process engineering. He then began his professional career in laboratory equipment distribution before joining the brand new Shimadzu France subsidiary in 2002. Since then, he has held various positions to develop the MS range business. Since September 2013, he is product manager for the MS range with Shimadzu Europe.
01.01.2020
- automation (182)
- cancer (861)
- chemotherapy (121)
- laboratory (1044)
- mass spectrometry (74)
- screening (173)